Infant ibuprofen sold at Walmart, CVS recalled over dosage concerns
MONMOUTH JUNCTION, N.J. – A New Jersey-based pharmaceutical company is recalling three lots of liquid infant ibuprofen that might be more concentrated than advertised.
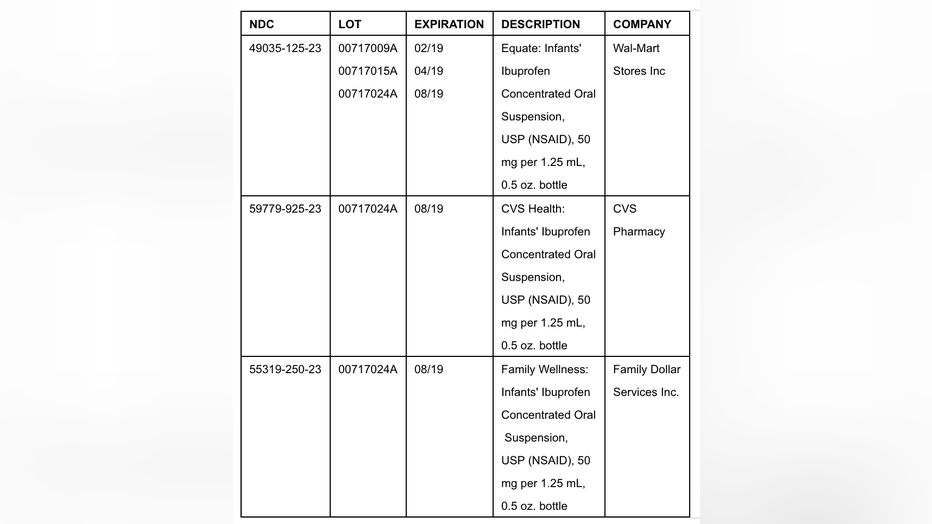
Tris Pharma's voluntary recall covers Infants' Ibuprofen Concentrated Oral Suspension, USP (NSAID) 50 mg per 1.25 ml.
The company says there is a "remote possibility" that infants more susceptible to medication with a higher potency of ibuprofen might suffer permanent renal injury.
"Adverse effects that may be experienced are nausea, vomiting, epigastric pain, or more rarely, diarrhea," according to Tris Pharma's press release Wednesday. "Tinnitus, headache and gastrointestinal bleeding are also possible adverse effects."
The lots of recalled product are listed below:
According to Tris Pharma, they have not received any reports of adverse effects.
Wholesalers and retailers should stop selling the affected lots and consumers with questions can call Tris Customer Service at 732-940-0358 (Monday through Friday, 8:00am ET- 5:00pm PT) or via email at Customer Service Email .

