FDA requires lower dosage for insomnia drugs
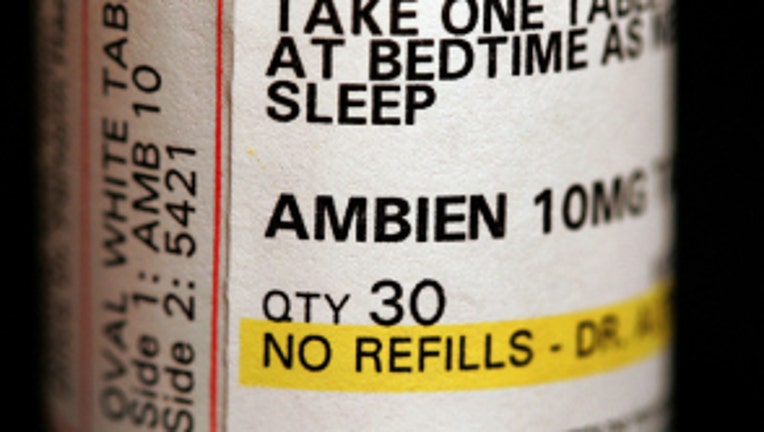
WASHINGTON -- The Food and Drug Administration announced Thursday it is requiring manufacturers of insomnia products, such as Ambien, Edluar and Zolpimist, to lower their current recommended doses.
The drugs contain an active ingredient called zolpidem, which causes drowsiness. Researchers have found high levels of zolpidem in the blood of some patients the morning after a dose -- so much so it affects patients' cognitive skills, including their ability to drive.
[trib_ndn vid=24207226]
The FDA said using lower doses of zolpidem will cut down on the amount of the drug found in the bloodstream the following morning. It also noted that the recommended dosage should be lower for women, because they don't process zolpidem out of their system as quickly as men.
The agency recommended the dosage of zolpidem for women be lowered to 5 milligrams from 10 milligrams for immediate-release products and to 6.25 milligrams from 12.5 milligrams for extended-release products. Patients who use the extended-release forms of these drugs have an even higher risk for next morning impairment, according to the FDA.
The FDA said new labeling on the insomnia products will let doctors know they should consider a lower dose for men, especially for those who are having difficulty concentrating the next morning. And all patients, the FDA said, should be told about the possible "morning after" risks.
-- CNN
To read the entire CNN article, click here.

